
The most advanced Legionella detection technology on the market
Accurate, rapid results – with viability differentiation
Veriflow Legionella is a molecular based assay for the presumptive detection of Legionella species in water and environmental swab samples without the need for enrichment. The Veriflow system utilizes a game-changing technology that combines proven diagnostic principles for microbial detection with innovative, first-in-class scientific approaches. The robust platform performs at the highest levels of accuracy even in the most challenging matrices, with vastly simplified sample preparation. The Veriflow system eliminates the need for gel electrophoresis, or fluorophore-based detection of target amplification. Results are visualized immediately on a hand-held cassette with no need for complex data analysis.
The Challenge
The increasing incidence of Legionella outbreaks, new regulatory guidance for maintaining water safety, and the recent state and federal mandates to enhance routine monitoring in healthcare facilities are driving an increase in testing for the presence of Legionella. Safety-conscious facility managers seek reliable and timely test results to ensure their patrons are not exposed to health risks. Yet traditional testing methods deliver results in 10-15 days, an unacceptably long duration in which organisms can potentially flourish in their habitat. Laboratories offering state-of-the-art testing services will set themselves apart as valued partners to their clients who demand accuracy and rapid turn-around for their routine water screening and environmental testing.
Veriflow Technology
Veriflow® technology is proven to provide rapid, accurate, actionable detection of Legionella – with no compromises on ease of use and affordability. Veriflow’s proprietary DNA Signature Capturing technology combines proven diagnostic principles for microbial detection with innovative, first-in-class scientific approaches for unparalleled performance. Veriflow technology is AOAC International Certified for foodborne pathogen detection, and has been widely adopted by third party testing labs and food manufacturers around the globe. Veriflow Legionella has passed CDC proficiency testing at CDC ELITE laboratories
Veriflow technology is easily deployed in the lab setting, allowing technicians to identify the presence of Legionella cells in under four hours from receipt of sample. A distinct advantage of the Veriflow DNA Signature Capturing method is the ability to accurately discern the presence of live versus dead cells, dramatically reducing the potential for false positive results.
Veriflow Legionella
Veriflow Legionella provides robust specificity and sensitivity for detection of live Legionella cells with minimal sample preparation.
Unmatched specificity
- Broad inclusivity of 13 Legionella isolates with 100% detection rate
- Correctly excludes dead cells as demonstrated by CDC proficiency testing
Unrivaled sensitivity
- Target amplification of a conserved gene marker for Legionella species
- Robust detection technology that is resistant to interference from inhibitory compounds that can be present in environmental samples
- Successfully validated with CDC proficiency samples by a leading ELITE certified testing laboratory
Unsurpassed value & ease of use
- Results in under 4 hours versus 10-15 days with traditional methods
- Simplified workflow and sample preparation with no filtration or enrichment steps required
- Modest capital investment and cost-effective consumables
- Veriflow capital equipment is compatible with entire suite of microbial detection assays from Invisible Sentinel
- Empowers early intervention (up to fifteen days earlier) to prevent infections and protect patrons
Performance Validation
CDC ELITE Proficiency Tested
The CDC’s (Center for Disease Control) Environmental Legionella Isolation Techniques Evaluation (ELITE) Program was utilized for validation and verification of assay performance at an ELITE member lab. In a blind study, proficiency samples provided by the CDC were evaluated comparing Veriflow Legionella to traditional culture methods. Veriflow Legionella correctly identified the presence or absence of Legionella in ALL samples and distinguished between viable and non-viable Legionella organisms. Veriflow Legionella provided 100% matched, accurate, and actionable results up to 15 DAYS EARLIER than the traditional culture-based method.
Test protocol
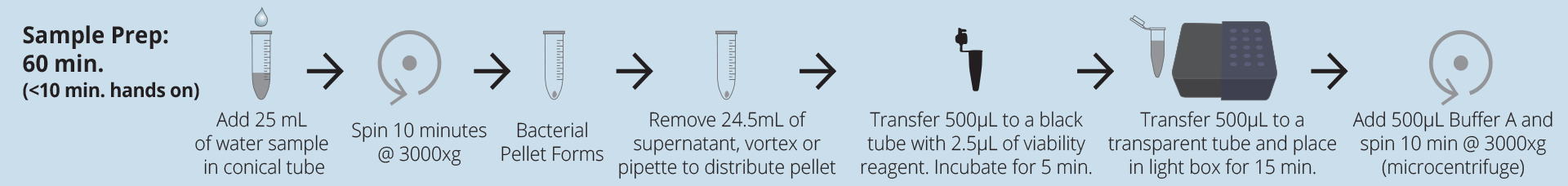
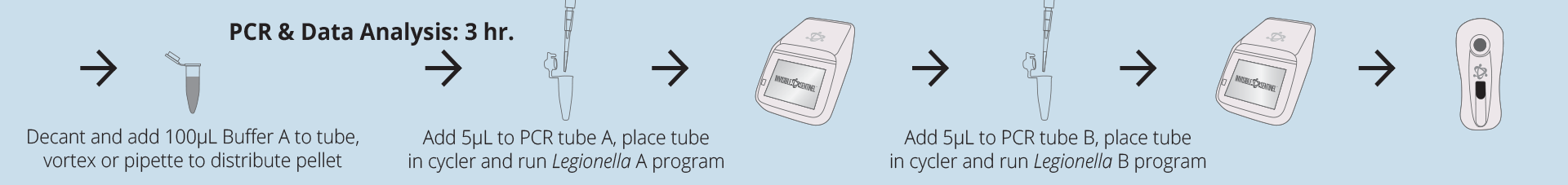
Technical specifications
Specificity
| Assay | Strains | Results |
|---|---|---|
| Inclusivity | 13 Legionella isolates: Legionella anisa, bozemanae,dumoffii, feeleii, jordanis, gormanii, longbeachae, micdadei, maceachernii, sainthelensi, pneumophila subsp. pneumophila, pneumophila subsp. pascullei, pneumophila subsp. fraseri |
100% Detection Rate |
| Exclusivity | 34 non-specific bacterial strains | 100% Exclusion Rate |
Sensitivity
| Matrix | Internal Validation | External Validation |
|---|---|---|
| Potable Water | YES | YES |
| Non-Potable Water | YES | YES |
| Rainwater | YES | YES |
| Chlorinated Water | YES | YES |
Performance Specifications
| Time to Results | 4 hours |
| Matrix Compatibility | Potable Water, Non-Potable Water, Rainwater, Chlorinated Water |
| Sensitivity (LOD) | 10 cells/mL |
| Assay Format | Qualitative |
| Test Stability | 1 year expiration with proper storage |
| Enrichment | Not required |
| Sample Preparation | Molecular platform that eliminates filtration and need for DNA extraction or purification |
| Cell Viability | Differentiates between live and dead cells |
| Workflow | 60 min. sample prep with less than 10 minutes of hands-on time |
| Results Interpretation | Immediate visualization on hand-held cassette – no complex data analysis |





